Product Registration & Production License
I. Main legal basis
Regulations for the Supervision and Management of Medical Devices
Measures for the Supervision and Administration of Medical Device Production
Measures for the Administration of Registration of Medical Devices
Announcement for Medical Device Registration Dossier Requirements and Certificate Template
Good Manufacturing Practice
II. Registration declaration
Certificate type:
Product registration certificate & production license
For the domestic Class II and III medical device products, the product registration certificate and the production license are both required before they can be formally produced and sold in China.
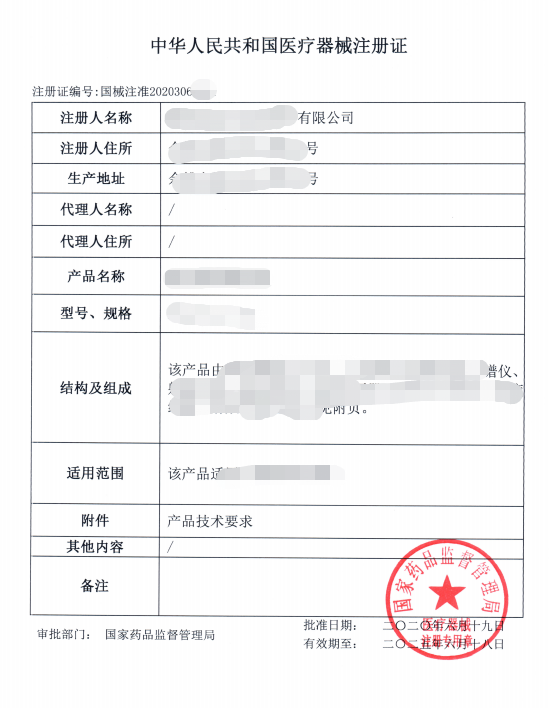
Example of medical device registration certificate
Example of medical device production license

Registration process:
To apply for the registration of the Class II medical device products, the registration applicants shall submit the registration application materials to the food and drug administration departments under the people’s governments of the provinces, autonomous regions, or municipalities directly under the Central Government where such applicants are located.
To apply for the registration of the Class III medical device products, the registration applicants shall submit the registration application materials to the food and drug administration department under the State Council.
For the imported Class II/III medical devices, the representative offices established or the corporate legal persons designated by the overseas manufacturers in China shall act as the agents, providing the food and drug administration departments under the State Council with the registration application materials as well as the documents proving that the competent authorities of the countries (regions) of the registration applicants approve the medical devices to be marketed.
The food and drug administration departments that accept the registration application shall forward the dossiers to the technical review agencies within 3 working days from the date of acceptance.
The technical review agencies shall complete the technical review of the registration of Class II medical devices within 60 working days, and complete the technical review of the registration of Class III medical devices within 90 working days.
System assessment:
The enterprise that applies for the registration of Class II/III medical devices shall establish and improve the quality management system compatible with the medical devices it produces and ensure the effective operation of the system in accordance with the requirements of the Good Manufacturing Practice for Medical Devices in combination with the product characteristics.
The inspection content involved in the GMP quality management system mainly includes the followings:
(I) Organization and personnel
(II) Plants and facilities
(III) Equipment
(IV) Document management
(V) Design and development
(VI) Purchasing
(VII) Production management
(VIII) Quality control
(IX) Sales and after-sales service
(X) Non-conforming product control
(XI) Adverse event monitoring, analysis and improvement
Certificate maintenance and update:
The validity period of the medical device registration certificate and production license is 5 years.
Renewal of registration:
If the medical device registration certificate expires and needs to be renewed, the registrant shall apply to the food and drug administration department for renewal of the registration 6 months before the expiration of the medical device registration certificate, and submit the dossiers in accordance with relevant requirements.
Change of registration:
If the product name, model, specification, structure, composition, scope of application, product technical requirements, production address of imported medical devices, etc. change, the registrant shall apply to the original registration department for the change of licensed items.
If the name and domicile of the registrant or the name and domicile of the agent are changed, the registrant shall apply to the original registration department for the change of registered items; if the production address of domestic medical devices is changed, the registrant shall change the registered items after the corresponding production license change.
III. Declaration documents
The product registration dossiers generally include the followings:
(I) Registration application form;
(II) Supporting documents;
(III) List of basic requirements for safety and effectiveness of medical device;
(IV) Overview documents;
(V) Research documents;
(VI) Manufacturing information;
(VII) Clinical evaluation documents;
(VIII) Product risk analysis documents;
(IX) Product technical requirements;
(X) Registration inspection reports;
(XI) Samples of Instructions for Use and labels;
(XII) Declaration of conformity.
The production license dossiers generally include the followings:
(I) Business license;
(II) Letter of authorization;
(III) Enterprise application report;
(IV) Production license application form;
(V) Product registration certificate;
(VI) Product technical requirements;
(VII) ID cards of the legal representative and the person in charge of the enterprise;
(VIII) Proof of the identities, academic qualifications and titles of the persons in charge of production, quality and technology;
(IX) List of the employees on the production management and quality inspection positions and their academic qualifications and titles;
(X) Documentary evidence of the production site;
(XI) Catalogue of main production equipment and testing equipment;
(XII) Quality manual and procedure documents;
(XIII) Process flow charts;
(XIV) Quality management system verification report.
IV. Service content
SUNGO can provide services related to the Chinese regulations, including:
· Develop the application solutions
· Make the medical device classification definition declaration
· Assist the enterprise to establish the GMP or GSP management system
· Prepare the medical device recording/registration dossiers
· Assist the enterprise to obtain the product recording/production recording certificates
· Assist the enterprise to obtain the product registration certificate/production license/operation license
· Assist in obtaining the product recording/registration certificates of imported medical devices
· Provide the training and counseling on medical device regulations
· Renewal of registration/change of licensed items/change of registered items
· Production license renewal/production license change
V. Service process
|
Process |
Specific task |
Division of work |
Cycle |
|
1 |
The enterprise provides the preliminary product information, and SUNGO determines the product classification and the declaration path |
Both parties |
2-3 working days |
|
2 |
SUNGO signs the cooperation agreement with the enterprise |
Both parties |
2-3 working days |
|
3 |
SUNGO assists the enterprise to establish a quality management system that meets the GMP |
Both parties; SUNGO provides the counseling in the whole course |
4-8 months |
|
4 |
Registration inspection |
Both parties; SUNGO guides the preparation of inspection materials and submission of the materials for inspection by the inspection institute, follows up the inspection progress, and assists in the rectification |
4-6 months |
|
5 |
Product registration declaration |
Both parties; with the support from the enterprise, SUNGO prepares the materials and completes the declaration |
3-4 months |
|
6 |
Acceptance by the NMPA |
Both parties; SUNGO assists in the rectification |
5-10 working days |
|
7 |
Technical review of registration by the NMPA |
NMPA |
3 months |
|
8 |
Obtaining the supplementary notice from the NMPA |
Both parties; SUNGO guides the completion of the rectification declaration |
1-3 months |
|
9 |
Review of supplementary documents by the NMPA |
NMPA |
3 months |
|
10 |
System inspection by the NMPA |
Both parties; with the support from the enterprise, SUNGO provides guidance for receiving the review |
2-3 working days |
|
11 |
System rectification |
Both parties; with the support from the enterprise, SUNGO guides the rectification and submits the relevant materials |
1-2 months |
|
12 |
Approval |
NMPA |
20 working days |
|
13 |
Certificate making |
NMPA |
10 working days |
|
14 |
After the end of the process, Class II/III medical devices can be produced |
N/A |
N/A |
