FDA Registration
U.S. agent
The U.S. regulations stipulate that each overseas enterprise exporting medical devices and food to the U.S. must identify a U.S. agent. The information of the U.S. agent shall be submitted electronically through the FDA Unified Registration and Listing System (FURLS system), which is a part of the establishment registration, and each foreign organization can only appoint one U.S. agent. SUNGO can provide you with U.S. agent service.
How to make SUNGO your U.S. agent?
After signing the U.S. agent service agreement, SUNGO will perform the duties of an U.S. agent for you and become a bridge between you and the FDA by virtue of its professionalism in the field of regulations.
After becoming your U.S. agent, what will SUNGO do for you?
· Colleagues of SUNGO in the U.S. will answer the FDA’s random telephone calls to the U.S. agent at any time;
· Receive the information or documents sent to you by the FDA or your U.S. customers and forward the same to you as soon as possible;
· Assist you in communicating with the FDA or U.S. customers, take advantage of SUNGO’s expertise in the U.S. regulations to help you, respond to relevant regulatory issues related to imports or providing imports to the U.S. as soon as possible, and avoid the FDA’s or U.S. customers’ concerns about your products during the supervision or sales, thereby helping you export and sell products smoothly.
· When the FDA conducts random inspection, assist you in communicating with the FDA’s U.S. inspection officers to determine the schedule for the factory audit, which usually needs to be communicated and confirmed within a week.
FDA establishment registration and device listing
All the enterprises related to the products sold to the U.S., including but not limited to manufacturers, OEMs, sterilization stations, and import and export companies, need to go through the establishment registration and product listing with the FDA before they can export their products to the U.S. The registration and listing need to be completed by the U.S. agent. After you entrust SUNGO as your U.S. agent, we will complete the establishment registration and device listing for you.
For the 510k exempt products, the establishment registration and device listing are conducted directly, and for the 510k products, the 510k declaration is required before conducting the establishment registration and device listing.
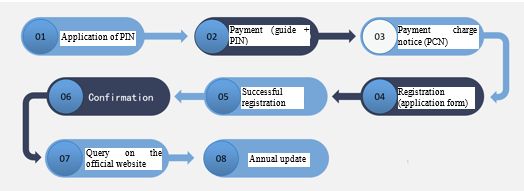
How do enterprises achieve FDA medical device registration?
· SUNGO will apply for you an PIN code (FDA official annual fee payment code)
· You should pay the official annual fee to the FDA according to the payment operation guide and PIN code given to you by SUNGO
· After receiving the payment notification and obtaining the PCN code, SUNGO will complete the establishment registration and product listing for you in the FDA system according to the application form you have filled out
· After successful registration, the FDA system will immediately generate the enterprise information, the Owner number issued by the FDA, and the listing number of the product. Such information will be displayed in the FDA’s electronic confirmation. SUNGO will hand the confirmation to you, and then the FDA will issue the registration number in the order of the application time.
· After successful registration, you will be able to inquire about your company information and product information on the FDA’s official website for registration information at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
· From October 1 to December 31 each year, you need to pay the FDA the official annual fee for the new fiscal year and update it in time to avoid the registration information being cancelled. SUNGO will contact you in advance to remind you to complete the annual update.
Tips:
1) The FDA will not issue any certificate. We will give you the officially downloaded electronic confirmation after successful registration. Before the registration number is issued, the Owner number can also be used for customs clearance.
2) The FDA official information query is updated on each Monday. If you do not find the information for the time being, please check again after next Monday.
FDA Food Establishment Registration
The food imported to the U.S. from overseas must meet the corresponding regulatory requirements as the food produced in the U.S. The food must be safe and contain no prohibited substances. All the labels and packages must clearly indicate the relevant real information in English.
Most of the food manufacturers, processing enterprises and packaging enterprises need to register, which is updated every two years.
This includes most of the overseas manufacturers and importers. There are some exceptions, such as farms and restaurants.
How do enterprises achieve FDA food registration?
· Fill in the FDA food registration application form.
· SUNGO will register your food in the FDA system according to the application form you provides.
· After successful registration, the FDA system immediately generates an electronic confirmation containing the enterprise information, the registration number issued by the FDA and other information (note that the FDA does not issue any certificate).
· It needs to be updated every even-numbered year, and SUNGO will contact you in advance and remind you to update it.
FDA cosmetics registration
For the cosmetics sold in the U.S., voluntary registration can be done with the FDA. Usually the registration contains 2 parts: establishment registration and product registration. In addition to cosmetic manufacturers, cosmetic packaging enterprises and dispensing enterprises can also apply for the registration.
After the FDA cosmetics registration, the enterprise will have an enterprise registration number and a product formula number (CPIS). The information that the enterprise needs to provide includes enterprise information (such as name, address, person in charge, and contact information) and product information (such as trademark, formula, and ingredient CAS number).
How long does the FDA cosmetics registration cycle take?
The materials will be ready in 2~3 weeks.
