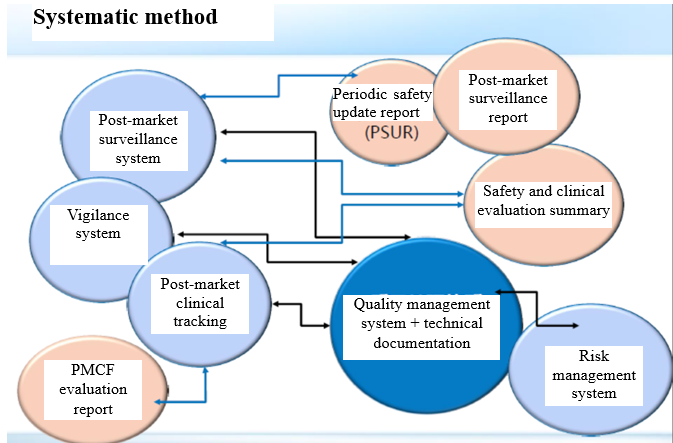
The MDR pays more attention to the post-market surveillance requirements, and the interested parties need to establish:
Post-market surveillance system;
Vigilance system;
Post-market clinical tracking system;
Risk management system.
Based on the above systems, considering the device risk category, it is required to form:
Post-market surveillance report;
Periodic safety update report;
PMCF report;
Safety and clinical performance summary.
All of these shall be reflected in the quality management system and technical documentation.