The technical documentation needs to be compiled using the STED structure.
STED is a guide prepared by the original GHTF for the structure of technical documentation. The structure specified in STED usually includes the followings:
· Device description
· Labeling and packaging
· Product design and production
· Basic requirements
· Risk analysis
· Verification and validation

Notes on technical documentation
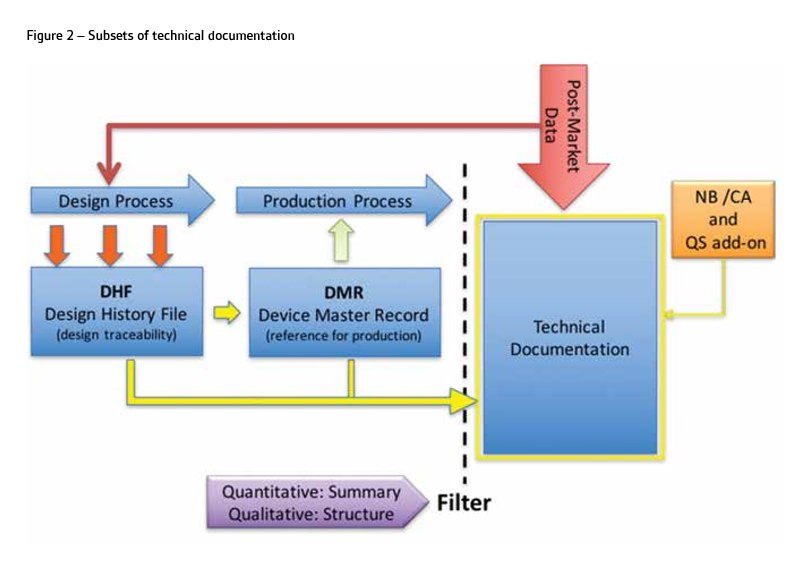
The technical documentation must be formed during the design and development of the device and maintained throughout the life cycle. As shown in Figure 1, this process can be represented by V-model because it delivers documents and records, which form design history files (DHF).
The technical documentation represents all documents describing the device. Therefore, it includes device design, development, V&V (including clinical and performance verification), and its regulatory position in the target market. In addition, the MDR now requires a closed-loop process from the marketing to the post-market surveillance (PMS), so as to ensure early warnings, continuous satisfaction of overall safety and performance requirements (GSPRs), and benefits to patients which always outweigh the risks.
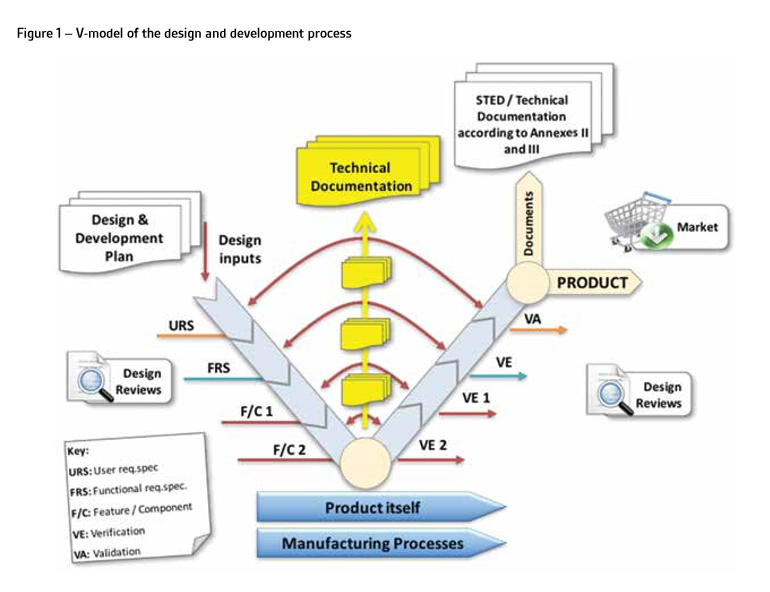
The technical documentation shall be presented in a structured way to facilitate the review and evaluation by the NB (Figure 2). This means that the preparation of technical documentation requires the application of quantitative and qualitative filters, allowing an appropriate level of detail to be maintained, while avoiding redundant details that are not necessary to prove the compliance with GSPRs.