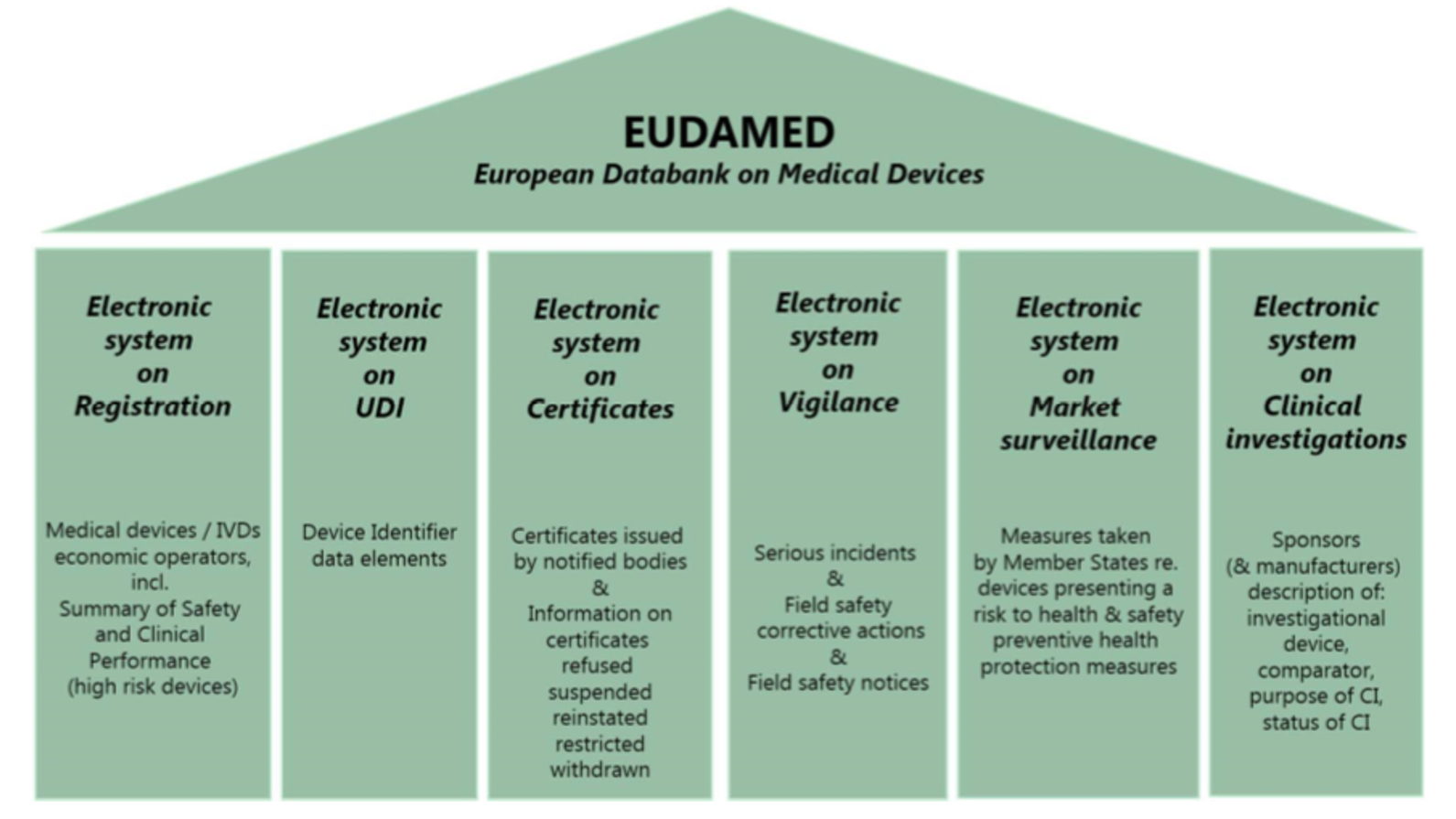
The Actors module is the first module that needs to be filled in. For each actor, specific data must be entered, such as name, address, and website. In addition, detailed information for identifying and contacting the person responsible for the regulatory compliance needs to be entered along with the actor’s data. These data of non-European manufacturers must also be entered, so they must apply for their SRNs with the competent authorities of the member states in which they are located and register their LUAs there. Their ARs need to be registered with Eudamed first. For the initial registration, there is no need to enter the name of the non-European manufacturer represented by the AR. In the next step, non-European manufacturers specify the ARs during their registration process, after which the ARs verify the registration process.
SRN-Single Registration Number
LUA-Local User Administrator