
Legal basis
21 CFR part 830 Unique Device Identification
· Definition Labeler
· UDI requirements
· FDA approved issuing agency
· Global Unique Device Identification Database (GUDID)
– Labeler information (name, contact person, issuing agency)
– Device version/model
DI, previously assigned DI (the identifier of new device replaces the original identifier), device direct label, brand name, version/model, sterile or not, natural latex-containing or not, MRI claimed or not, size, production identifier category, PMA/510K, listing number, GMDN code, device quantity
21 CFR part 801 Labeling
Subpart B--Labeling Requirements for Unique Device Identification
· The label of the medical device shall comply with the unique device identification (UDI) requirements
· Exception
– Finished devices produced and marked before the compliance date
– Class I devices exempted from GMP
– Independent disposable devices
– Devices for research, teaching or analysis
– Custom devices
– Survey devices
– Veterinary devices
– Exported devices
– Devices held by the national strategic reserve
– Exceptional devices recognized by regulations
– Devices packaged in combination products or convenience kits
– National Drug Code (NDC)-combination products
– Shipping package
– UDI for Class I devices does not need to contain a production identifier
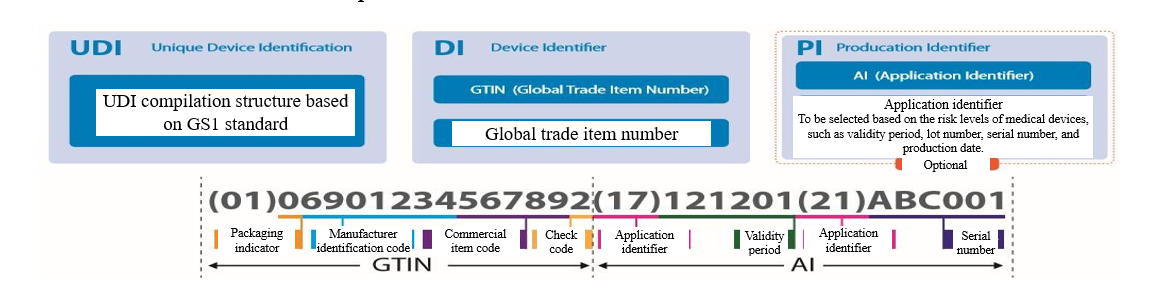
UDI structure
UDI – Unique Device Identification
UDI is a series of numeric or alphanumeric characters created through globally accepted device identification and coding standards. UDI is composed of UDI - DI and UDI - PI.
Device Identifier (DI) - a mandatory fixed part of UDI, used to identify a specific version or model of a medical device.
Production Identifier (PI) - a conditionally variable part of UDI, when included on the device label, used to identify one or more of the followings:
1. Lot number of a produced device
2. Serial number of a specific device
3. Expiration date of a specific device
4. Production date of a specific device
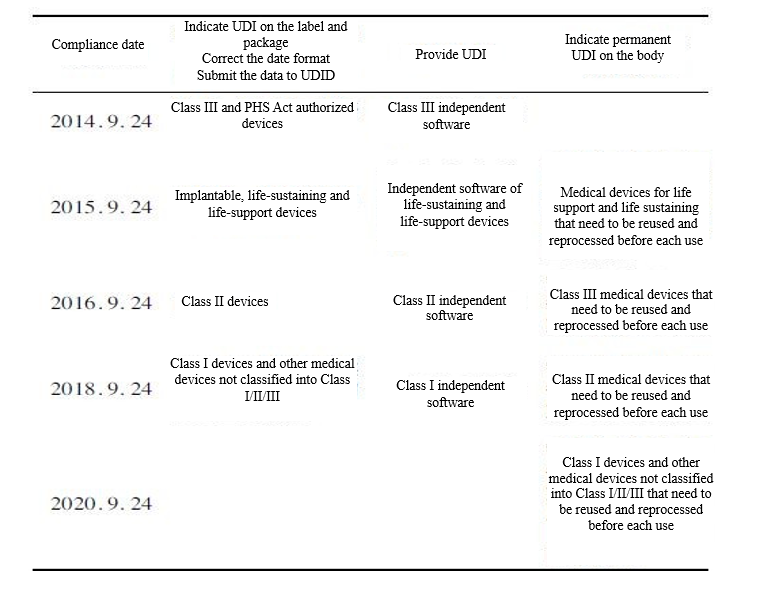
UDI compliance date
GUDID application process
1. Identify the Labeler;
2. Prepare before applying for an account: apply for a DUMS Number (Dun & Bradstreet Number) and a valid FDA Premark Number (510k or PMA, etc.), select a notified body, and apply for its barcode;
3. Fill in the “FDA Global Unique Device Identification Database (GUDID) New Account Request” and apply for a GUDID account by email;
4. Submit the information on GUDID. After the necessary information is completed, it will be announced on the release date. In principle, it cannot be modified after release.
5. Core elements of GUDID

