
1. Applicability of CE MDR/IVDR
The EU MDR and IVDR will enter into force on May 26, 2021 and May 26, 2022 respectively. The devices sold to Northern Ireland still need to bear the CE mark and meet the EU rules even after July 1, 2023. The CE certificates issued by the UK NB before January 1, 2021 will no longer be recognized by the NI market after that date.
2. Applicability of UKCA
The UK approved institutions will be able to carry out the NI market conformity assessment (UK MDR 2002) in accordance with the directive, and the UK (NI) mark may be affixed but cannot replace the CE mark. The products with both the CE mark and UK (NI) mark cannot be put on the EU market. The devices with the UKCA mark will not be accepted in the NI market unless they bear the CE mark or CE UK (NI) mark.
3. What kind of product mark will take you to which market?
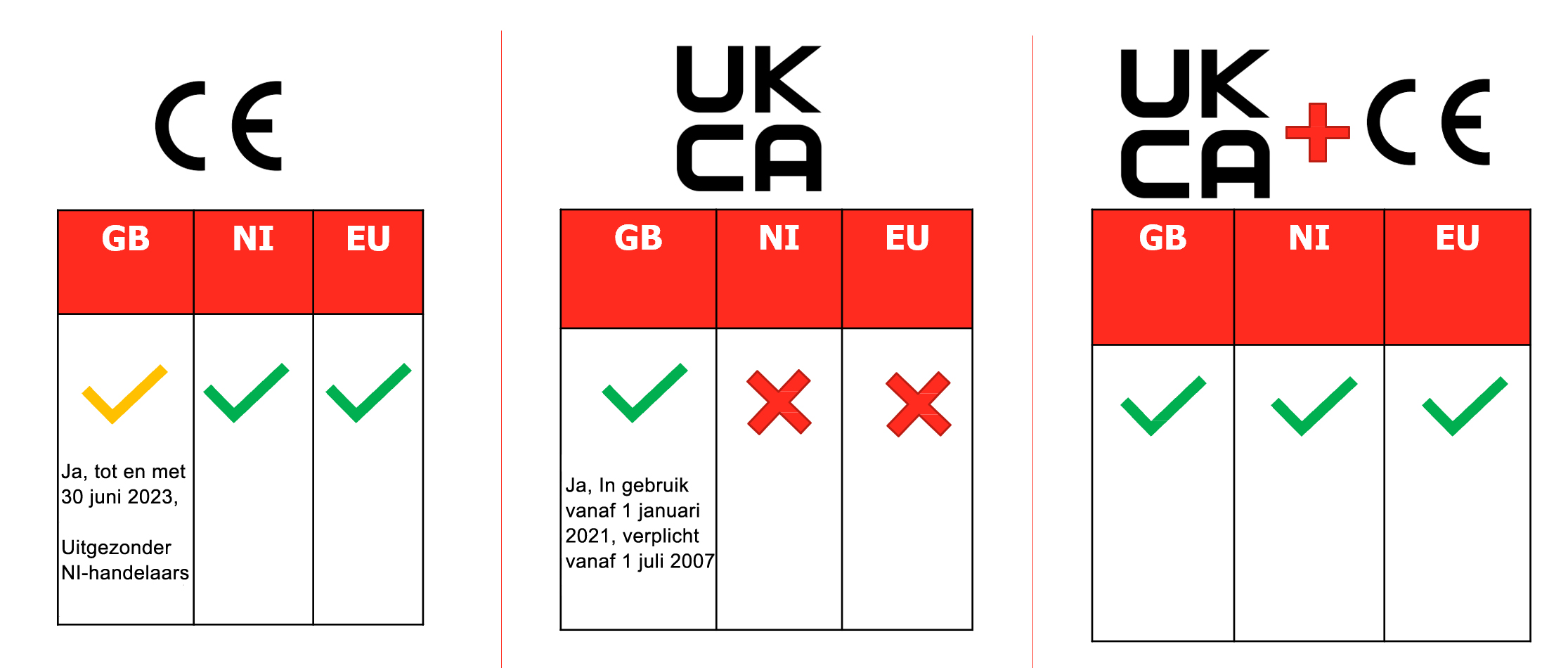
4. Requirements for the European Authorized Representative and the UK Representative
For the manufacturers who need to sell the medical device products to Northern Ireland:
If you are outside the EU, you need to designate a European Authorized Representative or UK Responsible Person in Northern Ireland;
If you are located in a member state of the EU, you need to designate a UK Responsible Person;
If you are located in the UK (except Northern Ireland), you need to designate an European Authorized Representative;
If you are located in Northern Ireland, you do not need to designate any representative.
