
1. Milestones for UKCA mark
It will be applicable from January 1, 2021.
It must be used to put the devices into the GB market from July 1, 2023; but this is not applicable for the NI traders.
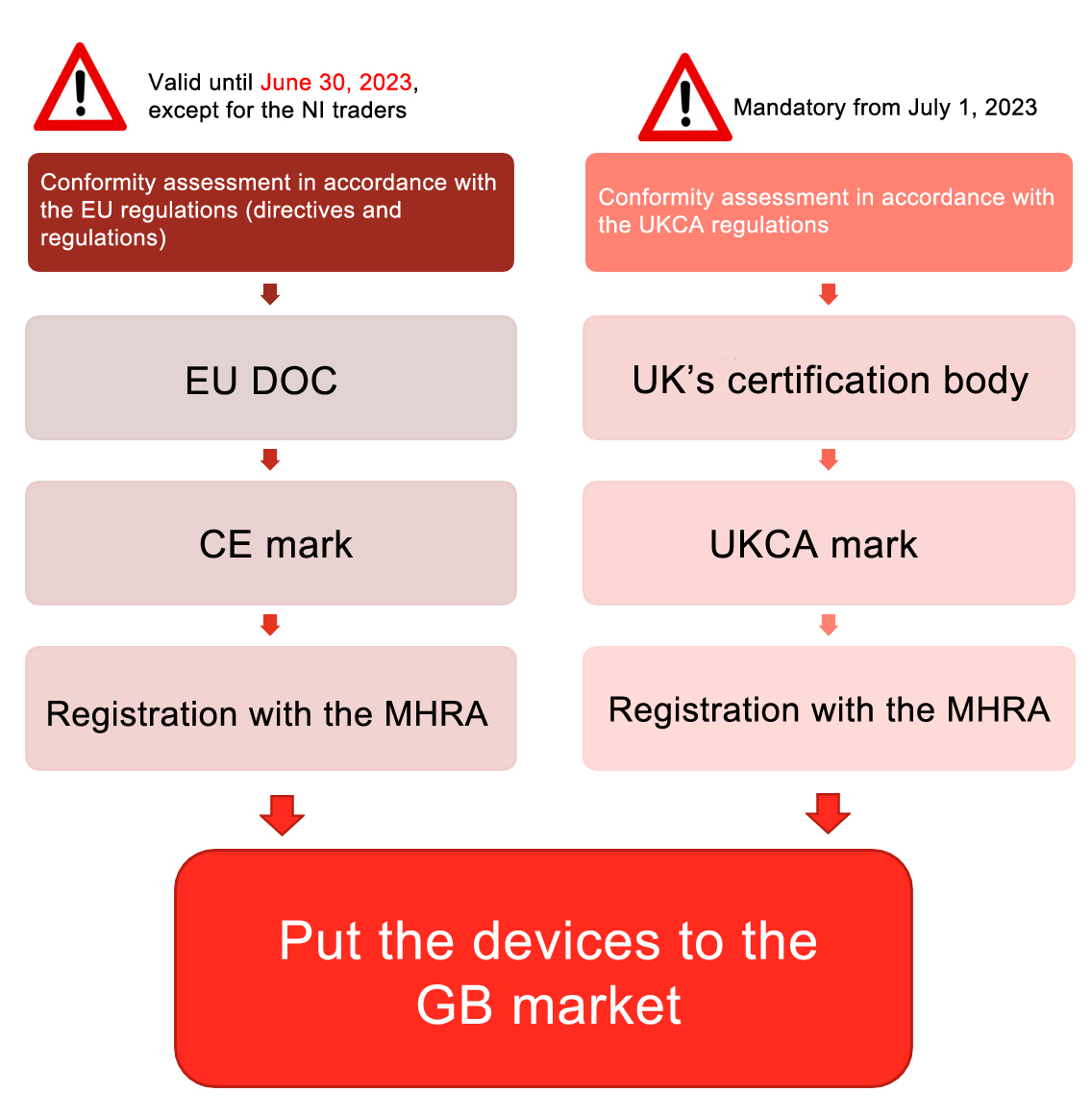
2. Conformity assessment procedure for the UKCA mark
The UK MDR 2002 review will be conducted by a UK accredited certification body, and the UKCA mark will be affixed after passing the review. In principle, the notified body of MDD/IVDD originally located in the UK can be directly converted into the UK accredited UK MDR 2002 certification body without the additional accreditation procedure.
The manufacturers of Class I medical devices and generic IVDs can declare their compliance with Parts II and IV of UK MDR 2002 (as of January 1, 2021), and then the UKCA marks can be affixed on the devices and the devices can be put on the UK market.
3. UKCA picture usage rules
You must ensure that:
• The picture is sized to scale;
• The height of the UKCA mark shall be at least 5mm - unless the relevant laws stipulate a different minimum size;
• The UKCA mark is clearly visible.

4. Affixing the UKCA Mark
In most cases, you must use the UKCA mark on the product itself or on the package.
In some cases, it may be placed in manuals or other supporting documents.
This will vary depending on the specific regulations applicable to the product.
The following general rules are applicable:
• The UKCA mark can only be affixed to the products by you as the manufacturer or your authorized representative;
• After the UKCA mark is affixed, you are fully responsible for the compliance of the products with the relevant regulatory requirements;
• You can only use the UKCA mark to indicate that the products comply with the relevant UK regulations;
• You shall not provide a third party with any mark or sign that may lead to the misunderstanding about the meaning or form of the UKCA mark;
• You shall not attach on the product other mark that affects the visibility, readability or meaning of the UKCA mark;
• Unless specially required by law, the UKCA mark shall not be placed on the products.
